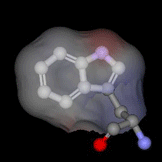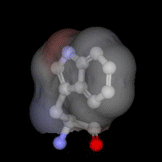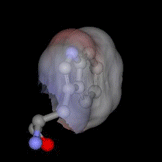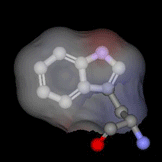
The highly buried, single tryptophan residue in the blue copper protein, azurin, is highlighted on the left (PDB 4AZU). Azurin continues to be an important system for the study of electron transfer reactions; as a result, we are utilizing this protein as a model system to study radical intermediates in long-range electron transfer reactions. An important aim of this research project is to elucidate the structure and kinetics of biological radicals, namely aromatic amino acid radicals, using an array of powerful spectroscopic tools. Despite the known significance of radicals to long-range ET, there is a dearth of knowledge on the structure and dynamics of the radicals. In addition, because there may be a dramatic shift in pKa upon radical formation, the protonation states of biologically-active radicals are often unknown, and fundamental questions regarding the nature of the proton-coupled electron transfer reaction are unresolved. Finally, the role of the protein microenvironment on the function and stability of radicals is unexplored. The goal of the current research project is to enhance our fundamental understanding of biological radicals in long-range ET. To do so, we will probe the structures and kinetics of aromatic amino acid radicals in controlled protein environments using a host of powerful time-resolved and steady-state optical and magnetic tools. Results from this project will not only enhance our understanding of how Nature has optimized proteins to perform otherwise unfavorable reactions, but may also advance our efforts in the design of efficient, long-range electron transfer scaffolds that may be applied, for example, to artificial photosynthetic materials. Return to main research page Read about resonance Raman spectroscopy |