|
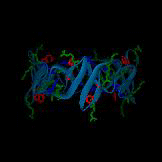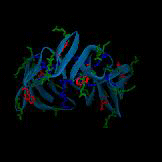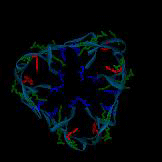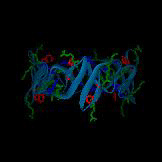
A postulated hexameric β-barrel structure of a human antimicrobial peptide in the defensins family,
HNP3, is shown on the left; this structure is composed of three dimer units (PDB 1DFN) and may be functionally relevant for membrane disruption.
In each monomer, the single tryptophan residue is shown in red, cationic residues are shown in green, and anionic residues are shown in blue.
The goal of our research is to probe molecular changes in antimicrobial peptide structure upon membrane association and subsequent membrane disruption.
The innate immunity of organisms in the plant and animal kingdoms relies upon ubiquitous antimicrobial peptides as the first line of
defense against pathogenic invasion. All antimicrobial peptides (AMPs) share a common electrostatic basis for recognition of pathogens:
AMPs are positively-charged (cationic) whereas microbial cell membranes are negatively-charged (anionic).
The fact that cell membranes of multicellular organisms are less anionic than their microbial counterpart helps explain the selectivity
of AMPs towards microbial membranes, but does not provide a mechanistic picture of how these natural peptide antibiotics invade cell membranes.
The potential for AMPs to constitute a novel class of therapeutic drugs that could combat antibiotic-resistant bacteria combined with the lack
of indepth, quantitative exploration of human AMPs motivates the current research proposal.
Our research involves the spectroscopic study of three human AMPs to (1) Identify functionally relevant, dynamic structures of active AMPs;
(2) Characterize residue-specific peptide-vesicle interactions to elucidate mechanisms of membrane disruption; and
(3) Determine oligomeric states in solution and membrane, and identify interfacial residues.
A suite of powerful spectroscopic techniques are utilized. Fluorescence and circular dichroism (CD) experiments shed light
on membrane permeability and overall secondary structure. Vibrational spectroscopy, specifically UV resonance Raman (UVRR) spectroscopy,
provides residue-specific, molecular detail while yielding information on changes in backbone structure associated with binding and insertion events.
Correlation spectroscopy complements these vibrational studies. A critical aim of this research project is to elucidate new quantitative,
molecular descriptions of AMP function to impact numerous aspects of human health, including the design of new peptide-based antibiotics
as well as implications for protection against growing biothreats.
Return to main research page
Read about resonance Raman spectroscopy
|